Neupro
What is Neupro (Rotigotine)?
Approved To Treat
Related Clinical Trials
Summary: This is a randomized, double-blind, placebo-controlled phase 2b pilot clinical trial to determine whether non-ergoline D3/D2/D1 dopamine (DA) receptor agonist rotigotine (RTG), in combination with treatment as usual, including individual or group behavioral therapy can a) reduce cocaine use and also b) increase brain activity in frontocortical areas of the brain, and, as a reflection of that - imp...
Summary: The study aims to observe changes in dopaminergic genes expression in peripheral tissue upon prolonged dopamine agonist treatment on patients with Restless Legs Syndrome (RLS). Similar studies in Parkinson's disease have shown changes in alpha-synuclein expression, which might offer insights into the dopaminergic gene regulation seen in RLS. The dopamine agonist drugs to be included in this study ...
Summary: This is a 24-week prospective, randomized, double-blind, placebo-controlled, multi-center phase III study evaluating efficacy and safety of rotigotine 4mg/24 hrs in combination with rivastigmine 9.5 mg/24 hrs in mild to moderate AD patients. The total study duration per patient from baseline to the end will be 24 weeks. The study has a placebo-controlled design to eliminate experimental biases tha...
Related Latest Advances
Brand Information
- Sulfite Sensitivity
- Falling Asleep During Activities of Daily Living and Somnolence
- Hallucinations/Psychosis
- Symptomatic Hypotension
- Syncope
- Impulse Control/Compulsive Behaviors
- Elevation of Blood Pressure and Heart Rate
- Weight Gain and Fluid Retention
- Dyskinesia
- Application Site Reactions
- Augmentation and Rebound in RLS
- Hyperpyrexia and Confusion
- Withdrawal Symptoms
- Fibrotic Complications
- have breathing problems including asthma.
- have daytime sleepiness from a sleep disorder or have unexpected or unpredictable sleepiness or periods of sleep.
- have mental problems such as schizophrenia, bipolar disorder, or psychosis.
- feel dizzy, nauseated, sweaty, or faint when you stand up from sitting or lying down.
- drink alcoholic beverages. This may increase your chances of becoming drowsy or sleepy while using NEUPRO.
- have high or low blood pressure.
- have or have had heart problems.
- are pregnant or plan to become pregnant. It is not known if NEUPRO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if NEUPRO passes into your breastmilk. The amount of breast milk you make may be decreased while taking NEUPRO. Talk to your doctor about the best way to feed your baby if you take NEUPRO.
- Read the Instructions for Use at the end of this leaflet for specific information about the right way to apply the NEUPRO patch.
- Use NEUPRO exactly as your doctor tells you to use it.
- NEUPRO comes in 4 different size (dose) patches for Parkinson's disease. Your doctor should start you on a low dose of NEUPRO. Your doctor will change the dose weekly until you are taking the right amount of medicine to control your symptoms. It may take several weeks before you reach the dose that controls your symptoms best.
- Apply NEUPRO 1 time each day at the same time each day.
- You may bathe, shower, or swim while wearing a NEUPRO patch. Water may loosen your NEUPRO patch.
- If the edges of the patch lift, you may tape them down with bandaging tape.
- If your NEUPRO patch falls off, apply a new NEUPRO patch for the rest of the day. The next day, apply a new patch at your regular time.
- If you miss a dose or forget to change your NEUPRO patch, apply a new NEUPRO patch as soon as you remember. Replace the NEUPRO patch at your normal time the next day.
- Do notstop using NEUPRO without talking to your doctor first. If your doctor tells you to stop using NEUPRO, you should ask your doctor for specific instructions on how to slowly and safely discontinue using NEUPRO. If you stop using NEUPRO, you may have withdrawal symptoms (see " ).
- Do notdrive, operate machinery, or do other dangerous activities until you know how NEUPRO affects you.
- Avoid exposing the site where you have applied your NEUPRO patch to heating pads, electric blankets, heat lamps, saunas, hot tubs, heated water beds, and direct sunlight. Too much medicine could be absorbed into your body.
- Do notuse NEUPRO during certain medical procedures called magnetic resonance imaging (MRI) or cardioversion. Using NEUPRO during these procedures could cause a burn to the site where you applied your NEUPRO patch.
- Avoid direct sunlight if you get a skin rash or irritation from NEUPRO until your skin heals. Sun exposure could lead to skin color changes.
- severe allergic reactions.NEUPRO contains a sulfite called sodium metabisulfite. Sulfites can cause severe allergic reactions that are life threatening to some people who are sensitive to sulfites. An allergy to sulfites is not the same as an allergy to sulfa. People with asthma are more likely to be allergic to sulfites. Remove your NEUPRO patch right away and call your doctor if you have swelling of the lips or tongue, chest pain, trouble breathing or swallowing.
- falling asleep during normal activities.You may fall asleep while doing normal activities such as driving a car, doing physical tasks, or using hazardous machinery while taking NEUPRO. You may suddenly fall asleep without being drowsy or without warning. This may result in having accidents. Your chances of falling asleep while doing normal activities while using NEUPRO are greater if you take other medicines that cause drowsiness. Tell your doctor right away if this happens. Before starting NEUPRO, be sure to tell your doctor if you take any medicines that make you drowsy.
- hallucinations and other psychosis.NEUPRO can cause or worsen psychotic symptoms including hallucinations (seeing or hearing things that are not real), confusion, excessive suspicion, aggressive behavior, agitation, delusional beliefs (believing things that are not real), and disorganized thinking. The chances of having hallucinations or these other psychotic-like changes are higher in people with Parkinson's disease who are elderly, taking NEUPRO, or taking higher doses of NEUPRO. If you have hallucinations or any of these other psychotic-like changes, talk with your doctor.
- changes in blood pressure.NEUPRO can decrease or increase your blood pressure. Lowering of your blood pressure is of special concern. If you faint or feel dizzy, nauseated, or sweaty when you stand up from sitting or lying down, this may mean that your blood pressure is decreased. If you notice this, you should contact your doctor. Also, when changing position from lying down or sitting to standing up, you should do it carefully and slowly. Lowering of your blood pressure can happen, especially when you start taking NEUPRO or when your dose is increased.
- fainting.Fainting can occur, and sometimes your heart rate may be decreased. This can happen especially when you start using NEUPRO or your dose is increased. Tell your doctor if you faint or feel dizzy.
- unusual urges.Some patients using NEUPRO get urges to behave in a way unusual for them. Examples of this are an unusual urge to gamble, strong urges to spend money, binge eating, or increased sexual urges and behaviors. Some patients may want to use more NEUPRO than prescribed for their symptoms (dopamine dysregulation syndrome). If you notice or your family notices that you are developing any unusual behaviors, talk to your doctor.
- changes in heart rate. NEUPRO can increase your heart rate.
- increased weight and fluid retentioncan occur in patients using NEUPRO. NEUPRO can cause your body to keep extra fluid which leads to swelling and weight gain. Tell your doctor if you have swelling or fluid retention, especially in the ankles or legs, or have an unusually fast increase in weight.
- uncontrolled, sudden movements.NEUPRO may cause uncontrolled, sudden movements or make such movements you already have worse or more frequent. Tell your doctor if this happens. The doses of your anti-Parkinson's medicine may need to be changed.
- skin site reactions.Skin reactions may occur at the site where you apply NEUPRO. Tell your doctor if you get a rash, redness, swelling, or itching that will not go away at the skin site where you have applied NEUPRO.
- withdrawal symptoms.NEUPRO is a dopamine agonist medicine. Dopamine agonist medicines, including NEUPRO, can cause withdrawal symptoms as your dose is slowly lowered (tapered) or when treatment with NEUPRO is stopped. Tell your doctor right away if you get any of the following withdrawal symptoms:
- fever
- confusion
- severe muscle stiffness
- feeling like you do not care things you usually care about (apathy)
- anxiety
- depression
- fatigue
- insomnia
- sweating
- pain
- Store NEUPRO at 68°F to 77°F (20°C to 25°C).
- Store NEUPRO in its original sealed pouch until use.

- 7 systems that deliver 1 mg/24 hours each
- Full Prescribing Information


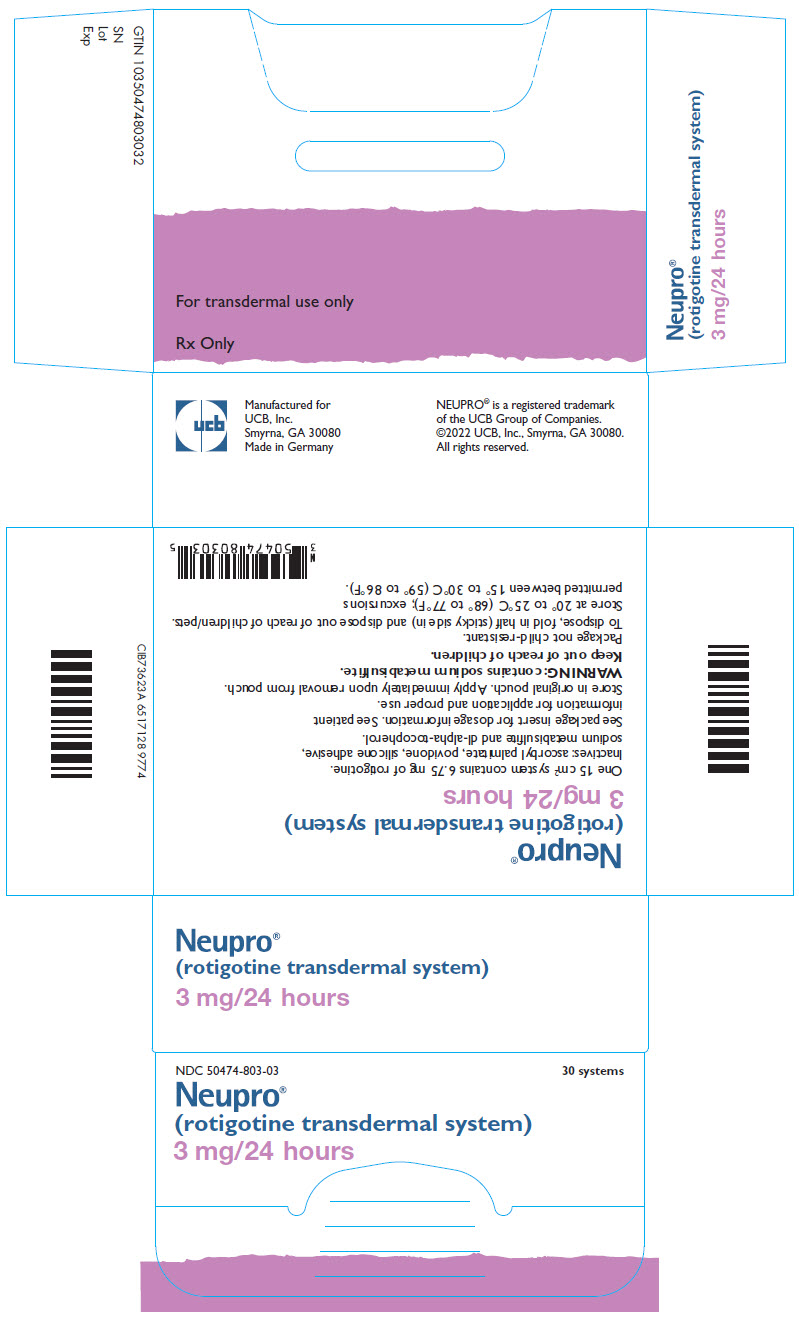

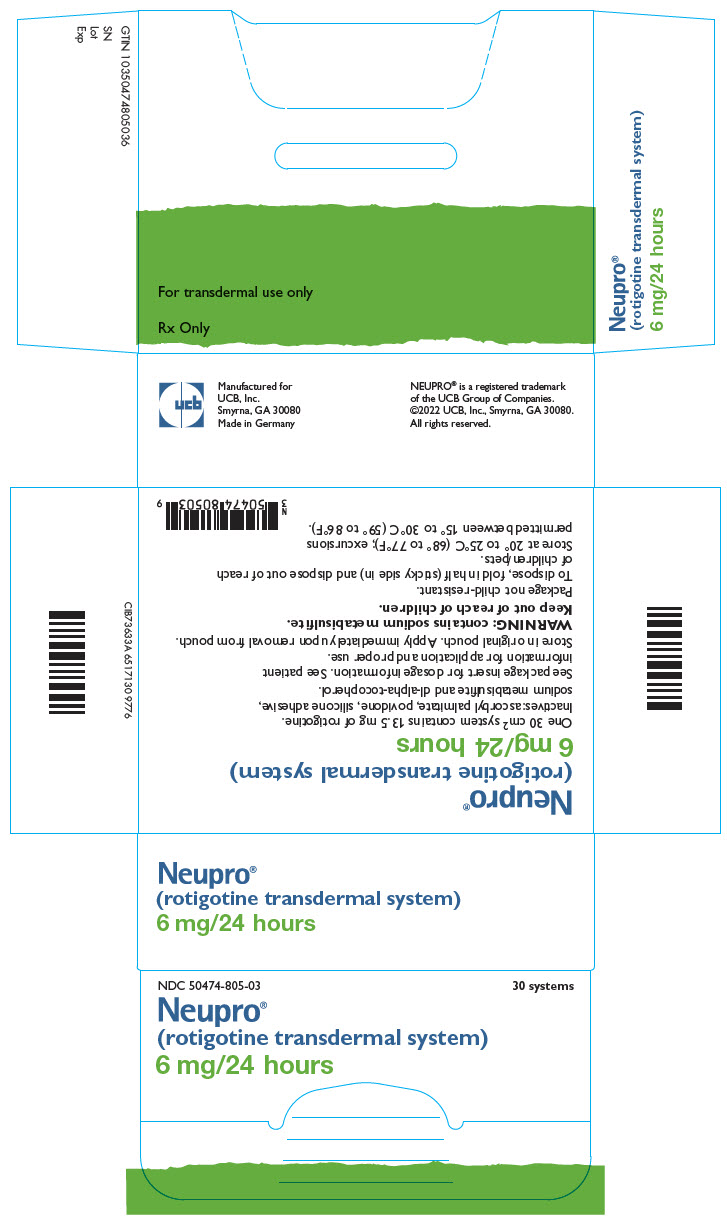

- 7 systems that deliver 2 mg/24 hours each
- 7 systems that deliver 4 mg/24 hours each
- Full Prescribing Information


